Bromine Oxide Oxidation State . Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Enter the formula of a chemical compound to find the oxidation number of each element. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. A net ionic charge can be specified. Web compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure for a given molecule.
from edurev.in
Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web compute formal charges for atoms in any lewis structure. Enter the formula of a chemical compound to find the oxidation number of each element. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. A net ionic charge can be specified. Use formal charges to identify the most reasonable lewis structure for a given molecule.
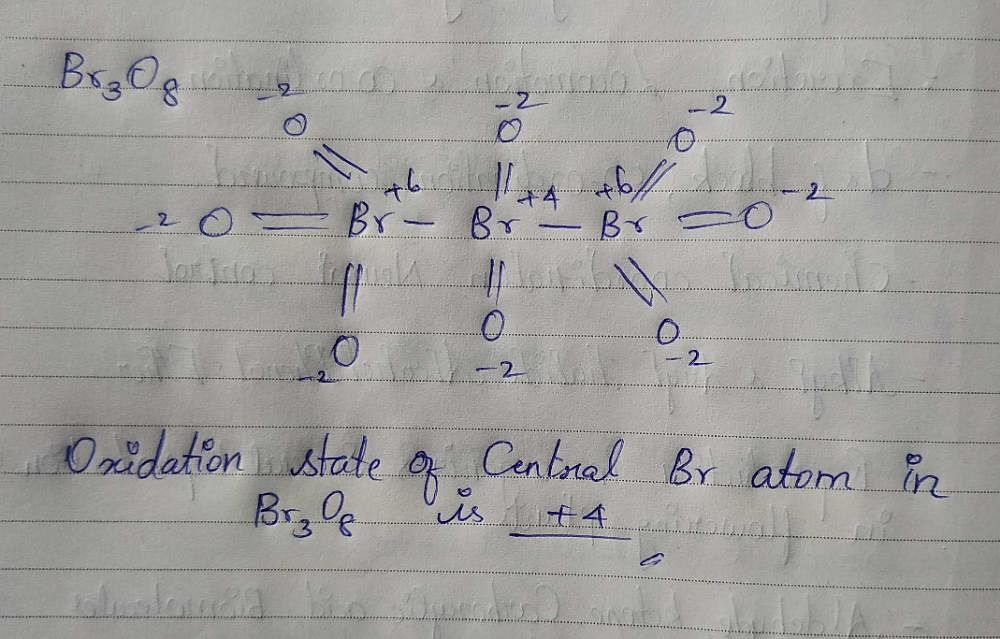
Oxidation state of Central Br atom in Br3O8 is? EduRev NEET Question
Bromine Oxide Oxidation State Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Use formal charges to identify the most reasonable lewis structure for a given molecule. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified. Web compute formal charges for atoms in any lewis structure. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation.
From www.numerade.com
SOLVED Why is HBrO the weakest acid when compared to HBrO2, HBrO3 and Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. A net ionic charge can be specified. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Enter the formula of a chemical compound to find the oxidation number of each element. Web the oxidation state of an atom is equal. Bromine Oxide Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Oxide Oxidation State Enter the formula of a chemical compound to find the oxidation number of each element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. A net ionic charge can be specified. Web the oxidation state of an atom is equal. Bromine Oxide Oxidation State.
From www.sarthaks.com
Consider the change in oxidation state of Bromine corredponding to Bromine Oxide Oxidation State Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web compute formal charges for atoms in any lewis structure. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Use formal charges to identify the most reasonable lewis structure for a. Bromine Oxide Oxidation State.
From www.chegg.com
Solved What is the change in oxidation state for the bromine Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Web compute formal charges for atoms in any lewis structure. Enter the formula of a chemical compound to find the oxidation number of each element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web the oxidation state of an. Bromine Oxide Oxidation State.
From askfilo.com
Consider the change in oxidation state of Bromine corresponding to differ.. Bromine Oxide Oxidation State Use formal charges to identify the most reasonable lewis structure for a given molecule. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Nonmetals tend to form covalent molecular bromides, as. Bromine Oxide Oxidation State.
From www.youtube.com
How to find the Oxidation Number for Br in BrO2 (Bromite ion) YouTube Bromine Oxide Oxidation State Use formal charges to identify the most reasonable lewis structure for a given molecule. A net ionic charge can be specified. Web compute formal charges for atoms in any lewis structure. Enter the formula of a chemical compound to find the oxidation number of each element. Web most metal bromides with the metal in low oxidation states (+1 to +3). Bromine Oxide Oxidation State.
From askfilo.com
8. Consider the change in oxidation state of Bromine corresponding to dif.. Bromine Oxide Oxidation State A net ionic charge can be specified. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Use formal charges to identify the most reasonable lewis structure for a given molecule. Nonmetals. Bromine Oxide Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Oxide Oxidation State Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. A net ionic charge can be specified. Web compute formal charges for atoms in any lewis structure. Use formal charges to identify. Bromine Oxide Oxidation State.
From www.shutterstock.com
Bromine Oxide Iii Chemical Formula Inside Stock Vector (Royalty Free Bromine Oxide Oxidation State Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Web compute formal charges for atoms in any lewis structure. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an. Bromine Oxide Oxidation State.
From www.quora.com
Does Chlorine and Bromine exhibit +4 and +6 oxidation state in oxides Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Web compute formal charges for atoms in any lewis structure. Enter the formula of a chemical compound to find the oxidation number of each element.. Bromine Oxide Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Oxide Oxidation State Use formal charges to identify the most reasonable lewis structure for a given molecule. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. A net ionic charge can be specified. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web the oxidation state of an atom is equal to. Bromine Oxide Oxidation State.
From edurev.in
Oxidation state of Central Br atom in Br3O8 is? EduRev NEET Question Bromine Oxide Oxidation State Enter the formula of a chemical compound to find the oxidation number of each element. Web compute formal charges for atoms in any lewis structure. A net ionic charge can be specified. Use formal charges to identify the most reasonable lewis structure for a given molecule. Web most metal bromides with the metal in low oxidation states (+1 to +3). Bromine Oxide Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Oxide Oxidation State Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web compute formal charges for atoms in any lewis structure. Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an. Bromine Oxide Oxidation State.
From cefsrmua.blob.core.windows.net
Bromine Compound Element at Mack Doucette blog Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. Web compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure for a given molecule. Web most metal bromides with the metal in. Bromine Oxide Oxidation State.
From kunduz.com
[ANSWERED] 1 Consider the change in oxidation state of bromine Kunduz Bromine Oxide Oxidation State Web compute formal charges for atoms in any lewis structure. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Use formal charges to identify the most reasonable lewis structure for a given molecule. A net ionic charge can be specified. Enter the formula of a chemical compound. Bromine Oxide Oxidation State.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Web the oxidation state of an atom is equal to the total number of electrons which. Bromine Oxide Oxidation State.
From askfilo.com
Consider the change in oxidation state of Bromine corresponding to differ.. Bromine Oxide Oxidation State Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. Web compute formal charges for atoms in any lewis structure. A net ionic charge can be specified. Web most metal bromides with the metal in low oxidation states (+1 to +3) are. Bromine Oxide Oxidation State.
From askfilo.com
92 Electrochemistry 8. Consider the change in oxidation state of Bromine Bromine Oxide Oxidation State A net ionic charge can be specified. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Use formal charges to identify the most reasonable lewis structure for a given molecule. Enter the formula of a chemical compound to find the oxidation number of each element. Nonmetals tend to form covalent molecular bromides, as. Bromine Oxide Oxidation State.